Platelets
Activation-specific platelet inhibitors
Platelet adhesion to arterial vascular lesions and plaques plays a key role in the complications of atherosclerosis leading to acute coronary syndromes (such as myocardial infarctions) as well as to ischemic cerebral strokes.
Dimeric glycoprotein (GP) VI-Fc (Revacept) is a human Fc fusion protein, which prevents the local activation of platelets at sites of vascular injury, acting like a “vascular coating”. This agent was originally conceived in a cooperation with the group of Meinrad Gawaz (Medical Clinic III of the University of Tübingen). Efficacy studies showed that Revacept resulted in significantly reduced thrombus formation at these sites. However, systemic hemostasis is not affected.
In a first in man study, all doses were well tolerated, no drug-related adverse events occurred, bleeding time was not prolonged. No bleeding complications nor platelet depletion (thrombopenia) were observed.
A clinical phase II study in patients with symptomatic carotid artery stenosis (transient ischemic attack (TIA) or stroke – NCT01645306; Eudra-CT 2011-001006-10) investigated cerebral infarctions/strokes by nuclear resonance tomography (DWI-MR), clinical results and blood parameters (e. g. platelet aggregation). Beneficial effects of Revacept, but no relevant adverse effects were observed (Uphaus et al., 2022): A combined clinical and safety endpoint was significantly improved with 120 mg Revacept, but not with 40 mg Revacept. The number of new peri-interventional infarctions in the brain (core lab analysed DWI-MR images) was reduced by 46% in the group treated with 120 mg Revacept compared to placebo (10% reduction with 40 mg Revacept).
Additionally, marker-labelled GPVI-Fc was used to detect instable plaques in patients with vascular disease in an innovative imaging method (PET-NMR) in a clinical study at the University of Tübingen.
Moreover, the Munich Heart Alliance of the German Centre for Cardiovascular Research (DZHK) performed the investigator-initiated ISAR-PLASTER trial on Revacept in coronary heart disease (NCT 03312855; EudraCT 2015-000686-32). The study protocol was published (Schüpke et al., 2019). The teams of Prof. Adnan Kastrati, German Heart Center, Munich and of Prof. Steffen Massberg, Großhadern Clinic of the Ludwig-Maximilian University, and further large clinical entities, who cooperate within the DZHK, showed pharmacodynamic proof of efficacy in the blood of the patients in aggregation measurements: on top of maximum conventional therapy, Revacept inhibited platelet aggregation specifically. However, the primary endpoint of death or myocardial injury was not reached, possibly because technical problems during stenting such as side branch occlusion cannot be addressed by the local effect of Revacept. The study was published in JAMA Cardiology (Mayer et al., JAMA Cardiol. 2021;6(7):753-761; doi:10.1001/jamacardio.2021.0475).

References
- Uphaus T, Richards T, Weimar C, Neugebauer H, Poli S, Weissenborn K, Imray C, Michalski D, Rashid H, Loftus I, Rummey C, Ritter M, Hauser TK, Münch G, Gröschel K, Poppert H. Revacept, an Inhibitor of Platelet Adhesion in Symptomatic Carotid Stenosis: A Multicenter Randomized Phase II Trial. Stroke. 2022: online ahead of print; STROKEAHA121037006. doi:10.1161/STROKEAHA.121.037006
- Mayer K, Hein-Rothweiler R, Schüpke S, Janisch M, Bernlochner I et al. Efficacy and Safety of Revacept, a Novel Lesion-Directed Competitive Antagonist to Platelet Glycoprotein VI, in Patients Undergoing Elective Percutaneous Coronary Intervention for Stable Ischemic Heart Disease. JAMA Cardiol. 2021;6(7):753-761; doi:10.1001/jamacardio.2021.0475
- Schüpke S, Hein-Rothweiler R, Mayer K, Janisch M, Sibbing D, Ndrepepa G, Hilz R, Laugwitz KL, Bernlochner I, Gschwendtner S, Kupka D, Gori T, Zeiher AM, Schunkert H, Massberg S, Kastrati A; ISAR-PLASTER-Trial Investigators. Revacept, a Novel Inhibitor of Platelet Adhesion, in Patients Undergoing Elective PCI-Design and Rationale of the Randomized ISAR-PLASTER Trial. Thromb Haemost. 2019;119(9):1539-1545
- Mojica Muñoz AK, Jamasbi J, Uhland K, Degen H, Münch G, Ungerer M, Megens R, Weber C, Lorenz R, Brandl R, Siess W. Recombinant GPVI-Fc added to single or dual antiplatelet therapy in vitro prevents plaque-induced platelet thrombus formation. Thromb Haemost. 2017; 117(8):1651-1659
- Reimann A, Li Z, Goebel S, Faßbender J, Holthoff HP, Gawaz M, Münch G, Ungerer M. Combined administration of the GPVI-Fc fusion protein Revacept with low dose thrombolysis in the treatment of stroke. Heart Int 2016, 11 (1): e10-e16.
- Jamasbi J, Megens RTA, Bianchini M, Münch G, Ungerer M, Faussner A, Sherman S, Walker A, Goyal P, Jung S, Brandl R, Weber C, Lorenz R, Elia N, Farndale J, Siess W. Differential inhibition of human atherosclerotic plaque- induced platelet activation by dimeric GPVI-Fc and anti-GPVI antibodies: functional and imaging studies. J Am Coll Cardiol 2015; 65 (22): 2404-2415
- Bigalke B, Phinikaridou A, Andia ME, Sunassee K, Cooper MS, Schuster A, Schönberger T, Onthank D, Ungerer M, Gawaz M, Nagel E, Botnar RM. PET/CT and MR Imaging in a Murine Model of Progressive Atherosclerosis Using 64Cu-labeled Glycoprotein VI-Fc. Circulation Cardiovascular Imaging 2013;6(6):957-64
- Ungerer M, Münch G. Novel antiplatelet drugs in clinical development. J Thromb Haemost 2013, 110(5):868-75.
- Ungerer M, Li ZM, Baumgartner C, Göbel S, Vogelmann J, Holthoff HP, Bültmann A, Gawaz M, Münch G. The GPVI – Fc fusion protein Revacept reduces thrombus formation and improves vascular dysfunction in atherosclerosis without any impact on bleeding times. PLOS ONE 2013; 8(8):e71193; doi 10.1371/journal.pone.0071193.
- Göbel
S, Li ZM, Vogelmann J, Holthoff HP, Degen H, Hermann DM, Gawaz M,
Ungerer M, Münch G. The GPVI-Fc fusion protein Revacept improves
cerebral infarct volume and functional outcome in stroke. PLOS One 2013; 8(7):e66960; doi 10.1371/journal.pone.0066960 - Bigalke B, Pohlmeyer I, Schönberger T, Griessinger CM, Ungerer M, Botnar RM, Pichler BJ, Gawaz M. Imaging of injured and atherosclerotic arteries in mice using fluorescence-labeled glycoprotein VI-Fc. Eur J Radiol 2011; 79(2):e63-9
- Schönberger T, Ziegler M, Borst O, Konrad I, Nieswandt B, Massberg S, Ochmann C, Jürgens T, Seizer P, Langer H, Münch G, Ungerer M, Preissner KT, Elvers M, Gawaz MP. The platelet collagen receptor GPVI-Fc reduces platelet adhesion to activated endothelium and preserves myocardial function after transient ischemia in mice. Am J Physiol (Cell Physiol) 2012; 303: C757-C766
- Ungerer
M, Rosport K, Bültmann A, Piechatzek R, Uhland K, Schlieper P, Gawaz M,
Münch G. The novel anti-platelet drug Revacept (dimeric GPVI-Fc)
specifically and efficiently inhibited collagen-induced platelet
aggregation without affecting general haemostasis in humans. Circulation
2011; 123: 1891-1899 - Bültmann A, Li Z, Wagner S, Peluso M, Schönberger T, Weis C, Konrad I, Stellos K, Massberg S, Nieswandt B, Gawaz M, Ungerer M, Münch G. Impact of glycoprotein VI and platelet adhesion on atherosclerosis–a possible role of fibronectin. J Mol Cell Cardiol 2010; 49(3):532-42
- Massberg S, Konrad I, Bültmann A, Schulz C, Münch G, Peluso M, Lorenz M, Schneider S, Besta F, Müller I, Hu B, Langer H, Kremmer E, Rudelius M, Heinzmann U, Ungerer M, Gawaz M. Soluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivo. FASEB J. 2004;18(2):397-9
- Bültmann A, Herdeg C, Li Z, Münch G, Baumgartner C, Langer H, Kremmer E, Geisler T, May A, Ungerer M, Gawaz M. Local delivery of soluble platelet collagen receptor glycoprotein VI inhibits thrombus formation in vivo. Thromb Haemost 2006: 95:763-766
Platelet-inhibiting antibodies
Platelet adhesion to arterial vascular lesions and plaques can also be inhibited by using antibodies which specifically block glycoprotein VI -dependent signal pathways.
Such monoclonal antibodies were designed in a cooperation with the group of Meinrad Gawaz (now: Medical Clinic III of the University of Tübingen) and Dr. Kremmer at Helmholtz Centre Munich, Germany. Efficacy studies showed that also these antibodies resulted in significantly reduced thrombus formation at these sites, but systemic hemostasis was not affected.
Also these antibodies including fully human variants have already been tested in preclinical studies for safety. The group of Prof. Siess at the institute for cardiovascular research at Munich investigated their efficacy by using human carotid surgery-derived plaques which were superfused with human blood, as published in the Journal of the American College of Cardiology.
The pros and cons of using such anti-glycoprotein VI antibodies versus glycoprotein VI fusion proteins are discussed in the recent editorial of Kleiman und Kolandaivelu „Expanding the Roster : Developing New Inhibitors of Intravascular Thrombosis“ in the Journal of the American College of Cardiology 2015; 65 (22): 2416 – 2419.
Fusion proteins with GPVI-Fc
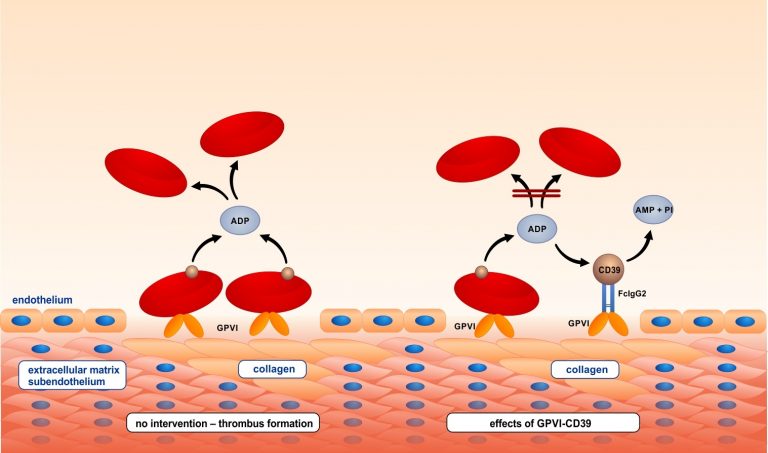
The platelet inhibitory potential of GPVI-Fc was further increased by fusing it to the ecto-nucleotidase CD39 which inhibits local adenosine diphosphate (ADP) accumulation at vascular plaques, and hence to create a lesion-directed dual antiplatelet therapy. GPVI-CD39 effectively stimulated local ADP degradation, and led to a significantly increased inhibition of plaque-induced platelet thrombus formation under arterial flow conditions.