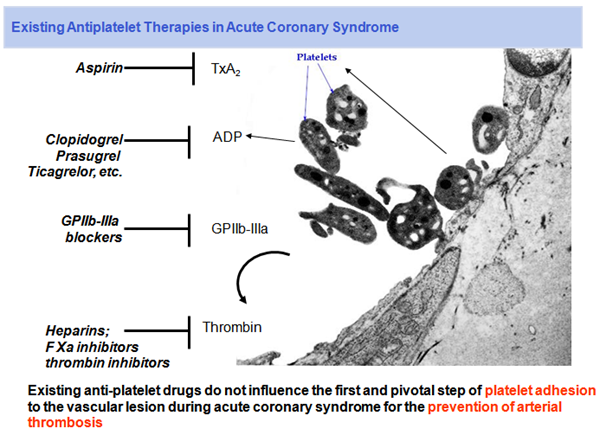
Therapeutical Principles
G protein-coupled receptors, receptor kinases and arrestins
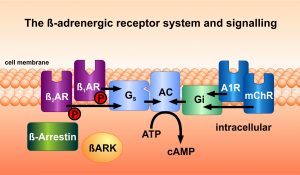
Adrenergic receptors play a pivotal role in the regulation of many physiological functions of the cardiovascular system. Catecholamines such as adrenaline and norepinephrine bind to these receptors which are common in many organs of the body. These receptors are activated by specific ligands, and can then be specifically inactivated by proteins which belong to the families of receptor kinases and of arrestins. The team has developed a number of compounds which bind to these proteins, and regulate the propagation of hormone stimuli to intracellular signalling pathways.
Additionally, the group of W. Koch and the 2012 Nobel prize awardee Robert J. Lefkowitz, in whose group Martin Lohse had worked for several years, investigated gene transfer of the C-terminal fragment „ßARKct“ of the ß-adrenergic receptor kinase-1 (ßARK-1, GRK-2) for its effect on heart muscle cells. They observed an improvement of myocardial function in heart failure in several animal models. Our team has extended these observations to an in vivo gene transfer model and to a related signalling protein, phosducin. New insight into the intracellular compartmentation of second messengers, especially cAMP, was obtained in cooperations. Methods to detect this signal activation and signal spreading optically on a millisecond scale were developed.
Specifically, novel assays were established which allow to determine molecular arrestin activation. Thereby, signal pathway-specific receptor agonists (“biased ligands”) can be identified.
References on receptor pharmacology and signalling
- Anton SE, Kayser C, Maiellaro I, Nemec K, Möller J, Koschinski A, Zaccolo M, Annibale P, Falcke M, Lohse MJ, Bock A. Receptor-associated
independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling. Cell 2022;185(7):1130-1142.e11. doi: 10.1016/j.cell.2022.02.011 - Bathe-Peters M, Gmach P, Boltz HH, Einsiedel J, Gotthardt M, Hübner H, Gmeiner P, Lohse MJ, Annibale P. Visualization of β-adrenergic
receptor dynamics and differential localization in cardiomyocytes. Proc Natl Acad Sci U S A. 2021;118(23): e2101119118. doi: 10.1073/pnas.2101119118 - Bock A, Annibale P, Konrad C, Hannawacker A, Anton SE, Maiellaro I, Zabel U, Sivaramakrishnan S, Falcke M, Lohse MJ. Optical Mapping of cAMP
Signaling at the Nanometer Scale. Cell 2020; 182: 1519–1530; e1–e17; doi: 10.1016/j.cell.2020.07.035 - Möller J, Isbilir A, Sungkaworn T, Osberg B, Karathanasis C, Sunkara V, Grushevskyi EO, Bock A, Annibale P, Heilemann M, Schütte C, Lohse
MJ. Single-molecule analysis reveals agonist-specific dimer formation of µ-opioid receptors. Nat Chem Biol 2020;16(9):946-954. doi:
10.1038/s41589-020-0566-1 - Annibale P, Lohse MJ. Spatial heterogeneity in molecular brightness. Nature Methods 2020;17(3):273-275. doi: 10.1038/s41592-020-0732-0
- Işbilir A, Möller J, Arimont M, Bobkov V, Perpiñá-Viciano C, Hoffmann C, Inoue A, Heukers R, de Graaf C, Smit MJ, Annibale P, Lohse
MJ. Advanced fluorescence microscopy reveals disruption of dynamic CXCR4 dimerization by subpocket-specific inverse agonists. Proc Natl Acad Sci US A 2020;117(46):29144-29154. doi: 10.1073/pnas.2013319117 - Grushevskyi EO, Kukaj T, Schmauder R, Bock A, Zabel U, Schwabe T, Benndorf K, Lohse MJ. Stepwise activation of a class C GPCR begins with
millisecond dimer rearrangement. Proc Natl Acad Sci U S A 2019; 116(20):10150-10155. doi: 10.1073/pnas.1900261116. - Sungkaworn T, Jobin ML, Burnecki K, Weron A, Lohse MJ, Calebiro D. Single-molecule imaging reveals receptor-G protein interactions at cell
surface hot spots. Nature 2017; 550(7677):543-547. doi: 10.1038/nature24264 - Godbole A, Lyga S, Lohse MJ, Calebiro D. Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription. Nature Commun. 2017;8(1):443
- Emami-Nemini A, Roux T, Leblay M, Bourrier E, Lamarque L, Trinquet E, Lohse MJ Time-resolved fluorescence ligand binding for G-protein-coupled receptors. Nature Prot 2013; 8: 1307-1320.
- Lohse MJ, Calebiro D. Receptor signals come in waves. Nature 2013; 495: 457-458.
- Calebiro D, Rieken F, Wagner J, Sungkaworn T, Zabel U, Borzi A, Cocucci E, Zürn A, Lohse MJ. Single-molecule analysis of fluorescently
labeled GPCRs reveals receptor-specific complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. USA 2013; 110:
743–748. - Hlavackova V, Zabel U, Frankova D, Bätz J, Hoffmann C, Prezeau L, Pin J-P, Blahos J, Lohse MJ. Sequential inter- and intra-subunit rearrangements during asymmetric activation of dimeric metabotropic glutamate receptor 1. Science Sign 2012; 5: ra59.
- Lohse MJ, Nuber S, Hofmann C. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor
activation and signalling. Pharm Rev. 2012; 64: 299–336. - Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol 2009; 7:e1000172.
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha 2A-adrenergic and mu-opioid
receptors controls cell signaling. Nature Chemical Biology 2008; 4: 126-131 - Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2-autophosphorylation causes cardiac hypertrophy. Nature Medicine 2009; 15: 75-83
- Nikolaev VO, Bünemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching ß1-adrenergic but locally confined alpha2-adrenergic receptor-mediated signaling. Circulation Research 2006; 99: 1084-1091.
- Buitrago M, Lorenz K, Maass AH, Oberdorf-Maass S, Keller U, Schmitteckert EM, Ivashchenko Y, Lohse MJ, Engelhardt S. The transcriptional repressor Nab1 is a specific regulator of pathological cardiac hypertrophy. Nature Medicine 2005; 11: 837-844.
- Hein P, Frank M, Hoffmann C, Lohse MJ, Bünemann M. Dynamics of receptor/G protein coupling in living cells. EMBO J. 2005; 24: 4106-4114.
- Hoffmann C, Gaietta G, Bünemann M, Adams S, Oberdorff-Maass S, Behr B, Vilardaga JP, Tsien RY, Ellisman MH, Lohse MJ. A FlAsH-based FRET
approach to determine G-protein coupled receptor activation in living cells. Nature Methods 2005; 2: 171-176. - Vilardaga JP, Steinmeyer R, Harms GS, Lohse MJ. Molecular basis of inverse agonism in a G protein-coupled receptor. Nature Chem. Biol. 2005; 1: 25-28.
- Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem 2004; 279: 37215-37218.
- Bünemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. USA 2003; 100: 16077-16082.
- Engelhardt S, Hein L, Dyachenkov V, Kranias EG, Isenberg G, Lohse MJ. Altered calcium handling is critically involved in the cardiotoxic effects of chronic ß-adrenergic stimulation. Circulation 2004; 109: 1154-1160
- Li Z, Laugwitz KL, Pinkernell K, Pragst I, Baumgartner C, Hoffmann E, Rosport K, Münch G, Humrich J, Lohse MJ, Ungerer M. Effects
of two Gbetagamma-binding proteins–N-terminally truncated phosducin and beta-adrenergic receptor kinase C terminus (ßARKct)–in heart failure. Gene Therapy 2003;10(16):1354-61 - Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature 2003; 426: 574-579
- Vilardaga JP, Bünemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G-protein-coupled receptors in
living cells. Nature Biotechnology 2003; 21: 807-812. - Ungerer M, Kessebohm K, Kronsbein K, Lohse MJ, Richardt G. Activation of ß-adrenergic receptor kinase during myocardial ischemia. Circulation Research 1996; 79: 455-460
- Ungerer M, Parruti G, Böhm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. Expression of ß-arrestins and ß-adrenergic receptor kinases in the
failing human heart. Circulation Research 1994; 74:206-213 - Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of ß-adrenergic receptor kinase (ßARK) and ß1-adrenergic receptors in
the failing human heart. Circulation 1993; 87:454-463 - Bauer PH, Müller S, Puzicha M, Pippig S, Obermaier B, Helmreich EJ, Lohse MJ. Phosducin is a protein kinase A-regulated G-protein regulator.
Nature 1992; 358(6381): 73-76 - Lohse MJ, Benovic J, Codina J, Caron MG, Lefkowitz RJ. ß-Arrestin: a protein that regulates ß-adrenergic receptor function. Science 1990;248: 1547-1550
Identification of novel therapeutic targets
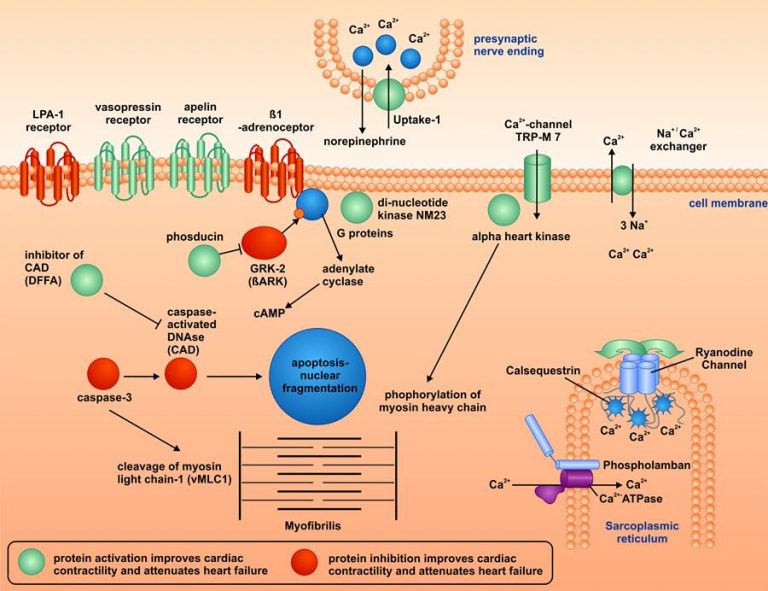
Gene transfer to the heart and cardiomyocytes
A broad-range screening program in relevant models of human cardiovascular disease has allowed to identify a number of novel targets in cardiovascular diseases – heart failure (by investigating heart muscle cells – cardiomyocytes), atherosclerosis (endothelial and vessel wall cells) and thrombosis (platelets).
The focus was on the direct, unbiased comparison of molecular interventions in various signalling pathways. This comparison tested for effects on disease as end points, but was not primarily driven by disease hypotheses.
Adenoviral or AAV-mediated gene transfer was used to directly test potentially relevant proteins in heart failure in rabbits which suffered from dilated cardiomyopathy. The method does not require any time consuming generation of transgenic mouse lines and no cross-breeding.
The effect on disease-relevant parameters was investigated by echocardiography and catheter-based intracardiac measurements of hemodynamics (method description in: Weig HJ, Laugwitz KL, Moretti A, Kronsbein K, Städele C, Brüning S, Seyfarth M, Brill T, Schömig A, Ungerer M. Enhanced cardiac contractility after gene transfer of V2 vasopressin receptors in vivo by ultrasound-guided injection or transcoronary delivery. Circulation. 2000; 101(13):1578-1585).
Among a larger number of potential target proteins, some especially relevant proteins were identified. The figure shows that activation of some proteins improved contractility and outcome of heart failure (marked in green), while inhibition of some others worsened both parameters (marked in red). These proteins belong to the families of activating receptors, of calcium-regulating proteins, of apoptosis-(cell death)-regulating proteins, or of contractile proteins. Further work demonstrated that agents which were developed to target these proteins hold promise for the treatment of patients with heart failure.
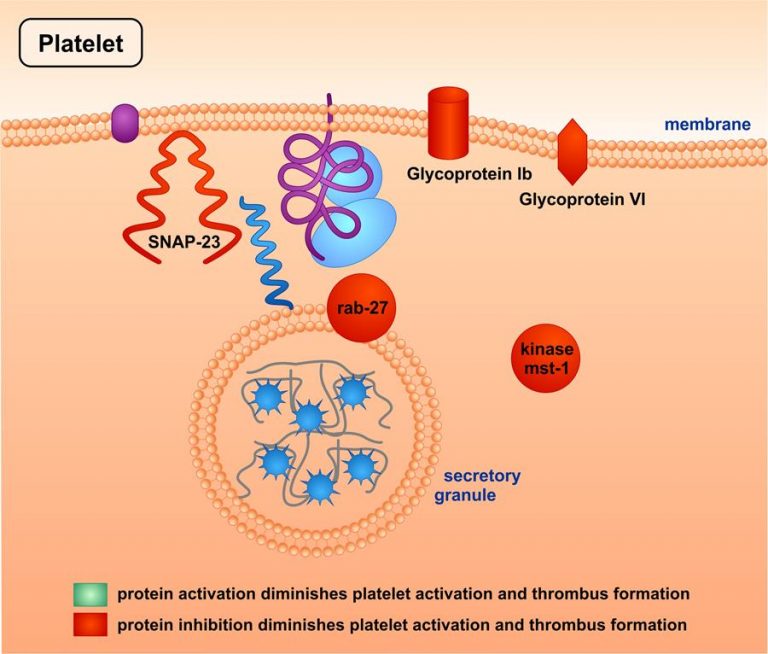
Gene transfer to megacaryocytes/platelets
Retroviral gene transfer to megakaryocytes resulted in recombinant platelets (thrombocytes) which could be harvested and used for experimentation ex vivo and vivo, e. g. measuring their activation, aggregation and secretion, as well as thrombus formation (method described in: Ungerer M, Peluso M, Gillitzer A, Massberg S, Schulz C, Heinzmann U, Münch G, Gawaz M. Generation of functional culture-derived platelets from CD34+ progenitor cells to study transgenes in the platelet environment. Circulation Research 2004; 95:e36-e44)
Among a larger number of potential target proteins, some especially relevant proteins were identified. The figure shows that activation of some proteins diminished platelet activation and thrombus formation (marked in green), while inhibition of some others worsened both parameters (marked in red). These proteins belong to the families of adhesion receptors, of secretion-regulating proteins, or of serin-/threonin kinases.
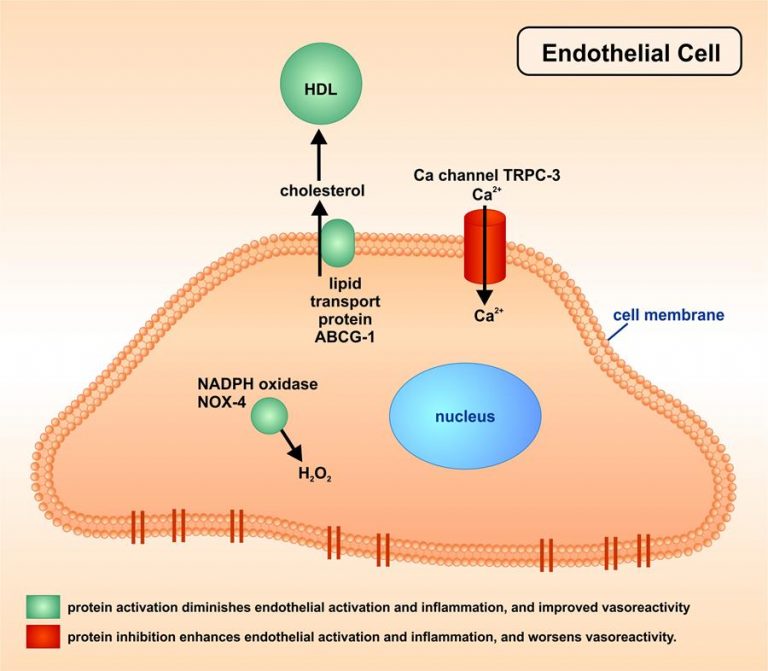
Gene transfer to the endothelium and wall of blood vessels
After adenoviral gene transfer to cultured human vascular endothelial cells and to atherosclerotic arteries in vivo activation of endothelial cells and vasoreactivity was tested after a volume or parasympathic challenge by ultrasound measurements in vivo (method described in in: Bültmann A, Herdeg C, Li Z, Münch G, Baumgartner C, Langer H, Kremmer E, Geisler T, May A, Ungerer M, Gawaz M. Local delivery of soluble platelet collagen receptor glycoprotein VI inhibits thrombus formation in vivo. Thromb Haemost 2006: 95:763-766).
Also these investigations showed that activation of some specific proteins (as outlined in the Figure) diminished endothelial activation and inflammation, and improved vasoreactivity (marked in green), while inhibition of some others worsened these parameters (marked in red). These proteins belong to the families of cholesterol transport proteins, of reactive oxygen-regulating proteins, or of calcium channels.
References
- Li Z, Laugwitz KL, Pinkernell K, Pragst I, Baumgartner C, Hoffmann E, Rosport K, Munch G, Moretti A, Humrich J, Lohse MJ, Ungerer M. Effects of two Gbetagamma-binding proteins–N-terminally truncated phosducin and beta-adrenergic receptor kinase C terminus (ßARKct)–in heart failure. Gene Therapy 2003;10(16):1354-61
- Bölck B, Munch G, Mackenstein P, Hellmich M, Hirsch I, Reuter H, Hattebuhr N, Weig HJ, Ungerer M, Brixius K, Schwinger RH. Na+/Ca2+ Exchanger Overexpression Impairs Frequency- and Ouabain-Dependent Cell-Shortening in Adult Rat Cardiomyocytes. Am J Physiol (Heart Circ Physiol.) 2004; 287:H1435-H1445
- Münch G, Rosport K, Bültmann A, Baumgartner C, Li ZM, Laacke L, Ungerer M. Cardiac overexpression of norepinephrine uptake-1 results in marked improvement of heart failure. Circulation Research 2005, 28;97(9):928-36.
- Gillitzer A, Peluso M, Bültmann A, Münch G, Gawaz M, Ungerer M. Effects of dominant negative inhibition of SNAP-23 on platelet function. J Thromb Haemost 2008; 6:1757-1763
- Münch G, Bültmann A, Li ZM, Holthoff HP, Ullrich J, Wagner S, Ungerer M. Overexpression of ABCG1 attenuates arteriosclerosis and endothelial dysfunction in atherosclerotic rabbits. Heart Int 2012; 7: e12 – e19
- Baumgartner C, Brandl J, Münch G, Ungerer M. Rabbit models to study atherosclerosis and its complications – transgenic vascular protein expression in vivo. Progr Biophys Molec Biol 2016; 121: 131-141
Therapy of receptor-directed auto-immune disease by „inverse vaccination“/hyposensitization
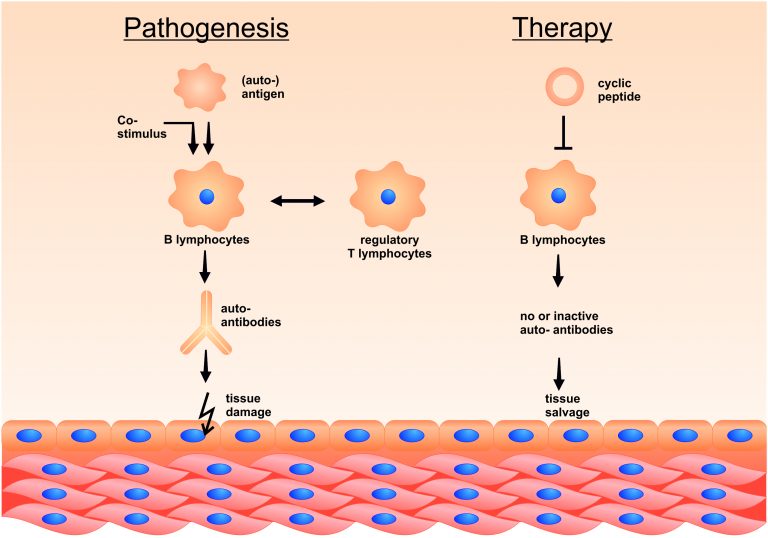
Autoimmune diseases are frequently caused by specific auto-antibodies, which are probably being generated in response to a false pattern recognition of “self“ surface structures as „alien“ and dangerous, e.g. because co-stimulatory signals are being recognized simultaneously by the immune system, or because of pathogen cross-reactivity.
Since many years, allergies have been successfully treated by hyposensitization (=specific immune therapy). During this therapy, increasing allergen doses are being injected subcutaneously, or (in a few cases) administered as sublingual medication. This therapy leads to consecutive symptom improvement. Sometimes, allergen preparations are not homogenous because they were extracted from crude extracts.
Accordingly, our team seeks for even more specific agents for such an “inverse” vaccination, which mimic relevant epitopes (i.e. the targets of antibodies) in their 3D structure, and are composed of natural amino acids. These agents are especially pure, and can therefore be used for the intravenous therapy of systemic auto-immune diseases.
Therapy of thrombo-embolic diseases by activation-specific platelet inhibition
Platelets play a pivotal role in many acute or chronic cardiovascular diseases. They are pivotally involved in the formation of arterial thrombi, which can then further embolise to peripheral tissues, and most damagingly, to the brain.
Such arterial thrombi originate from endothelial erosions and rupturing plaques, where subendothelial matrix is being exposed to the arterial blood. At such sites, collagen activates platelets. An important process during local platelet aggregation is direct activation of glycoprotein (GP) VI, and via collagen bound von Willebrand factor.